# Analysis of additional data
# February 21, 2020: New data is exceptionally sloppy
As of the morning of Feb 21, 2020 there are 11 additional datasets that appeared at the COVID-19 data page (opens new window) maintained by the NCBI (opens new window). They can be viewed using SRA run selector (opens new window).
The accessions of new datasets are:
SRR11085797
SRR11092056
SRR11085741
SRR11085737
SRR11085740
SRR11085738
SRR11085733
SRR11092064
SRR11092057
SRR11085736
SRR11092058
Of these four (SRR11092056, SRR11092057, SRR11092058, SRR11092064) are human metatranscriptomic samples while others (SRR11085797, SRR11085741, SRR11085737, SRR11085740, SRR11085738, SRR11085733, SRR11085736) are metatranscriptomic samples from bat anal swabs.
# New human data
First we analyzed the new human sequences represented by SRR11092056, SRR11092057, SRR11092058, SRR11092064. All these datasets share the same bioproject id PRJNA605983 (opens new window). The PRJNA605983 lists a total of nine SRA accessions: five more in addition to the four listed above:
SRR11092056
SRR11092057
SRR11092058
SRR11092059
SRR11092060
SRR11092061
SRR11092062
SRR11092063
SRR11092064
We attempted to download and analyze these data. It was only possible to download seven datasets from NCBI dues to repeated connection issues:
SRR11092056
SRR11092057
SRR11092058
SRR11092060
SRR11092061
SRR11092063
SRR11092064
We first processed reads using fastp to remove adapters and low quality reads. We then mapped reads against NC_045512.2 (opens new window) using bwa mem and retained only reads mapped in consistent forward/reverse configuration. The following numbers of reads were retained:
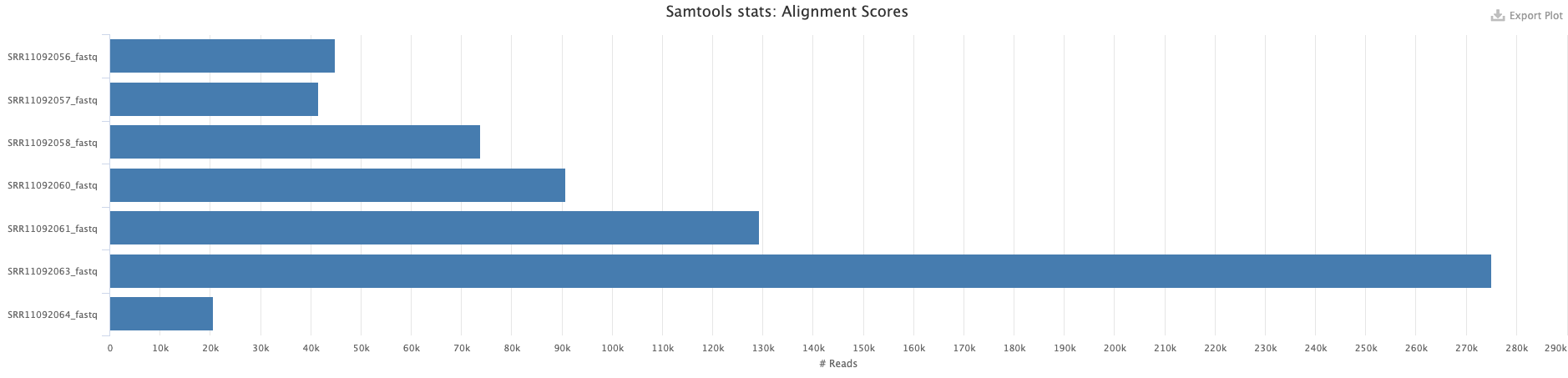
# Mapping statistics obtained with samtools stats and multiqc.
However, the distribution of reads across genome is extremely uneven as can be seen from the following analysis performed with deetTools:
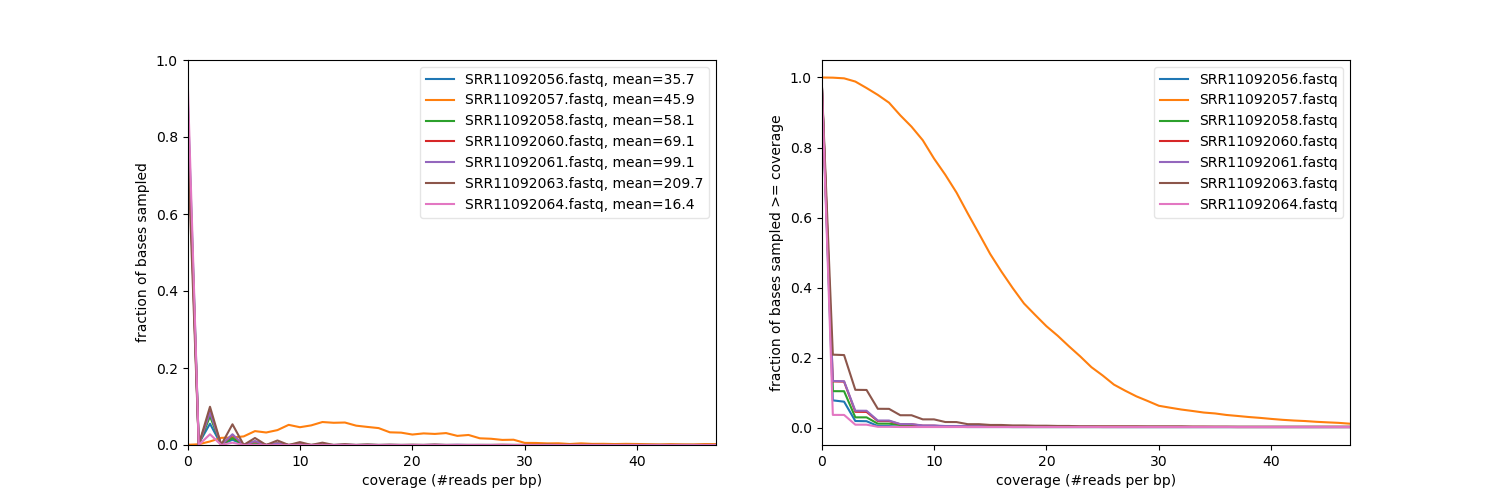
# Coverage analysis performed with deepTools: plotCoverage (opens new window).
it indicates that with the exception of sample SRR11092057 other samples essentially contain no mapped reads aside from one location at the very end of the COVID-19 genome:
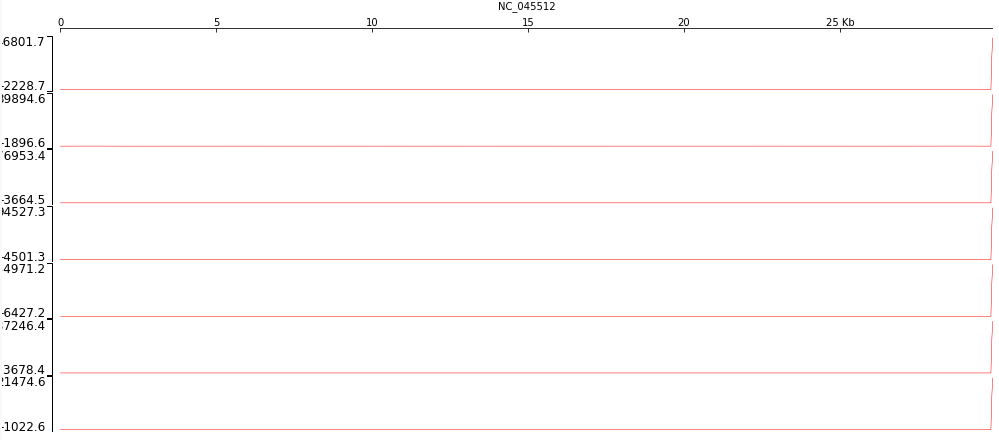
# Each panel represents one sample (SRR11092056, SRR11092057, SRR11092058, SRR11092060, SRR11092061, SRR11092063, SRR11092064). The coverage is flat at almost 0 until the the very 3' where it spikes sharply.
Here is a zoomed out view of the 3' region for sample SRR11092056 containing absolute majority of mapped reads:
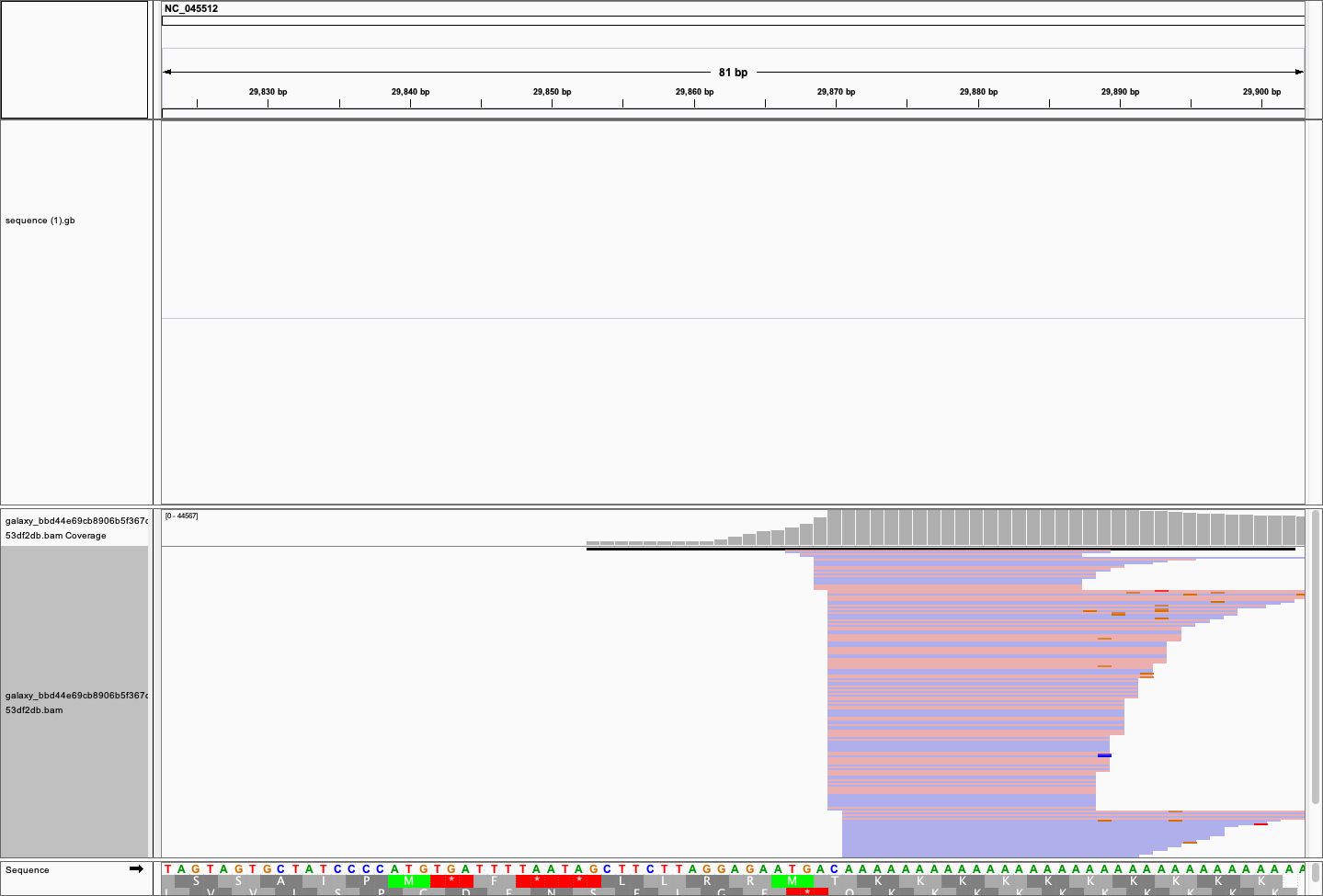
# An IGV screen-shot showing reads piling at the 3'-end of COVID-19 genome.
# The bottom line
New datasets released on Feb 21, 2020 do not contain meaningful COVID-19 sequence data. In addition, the metadata describing samples is highly questionable. For example, look at the description of another (this time non-human) dataset SRR11085740 (opens new window) generated by the same group (WUHAN INSTITUTE OF VIROLOGY, CHINESE ACADEMY OF SCIENCES):
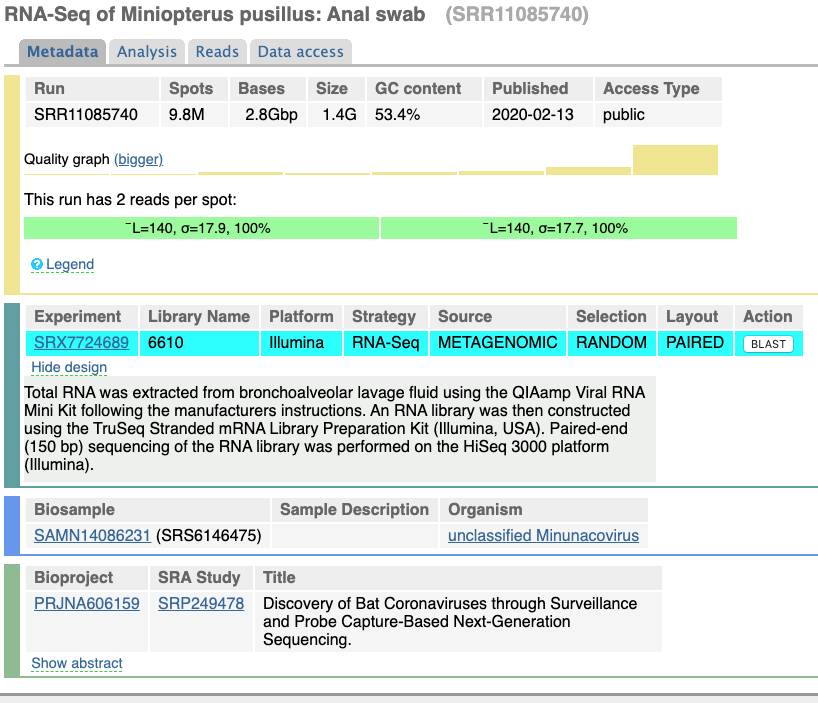
Note that while the description of the dataset lists "RNA-Seq of Miniopterus pusillus: Anal swab" the design field contains the following description:
Total RNA was extracted from bronchoalveolar lavage fluid using the QIAamp Viral RNA Mini Kit following the manufacturers instructions. An RNA library was then constructed using the TruSeq Stranded mRNA Library Preparation Kit (Illumina, USA). Paired-end (150 bp) sequencing of the RNA library was performed on the HiSeq 3000 platform (Illumina).
This leaves the question of whether the sample is derived from a bat anal swab of human bronchoalveolar lavage fluid.
# February 25: New datasets from University of Wisconsin
On February 25 we located new data generated by University of Wisconsin - Madison. They are not listed on NCBI's COVID-19 data page (opens new window) (UPDATE → As of Feb 28 these datasets are now listed). There are eight datasets:
SRR11140744
SRR11140745
SRR11140746
SRR11140747
SRR11140748
SRR11140749
SRR11140750
SRR11140751
They are produced by Katarina Braun (graduate student in Thomas Friedrich's lab; Twitter: @KATarinambraun; GitHub: @katarinabraun (opens new window)) and Gage Moreno (graduate student in Dave O'Connor's lab; Twitter: @GageKMoreno). Both are from the University of Wisconsin, Madison
This is a description of the samples as specified here (opens new window):
To characterize within-host viral diversity of this emerging virus, we obtained four SARS-CoV-2 samples from Yoshihiro Kawaoka’s lab: A clinical swab obtained from a confirmed case in Madison, WI. Cell culture isolates - all having undergone one passage each: Vero 76; ATCC CRL-1587 Vero E6, C1008; ATCC CRL-1586 Vero STAT-1 KO; ATCC CCL-81-VHG
From these we analyze the following four Illumina datasets (the other four are Oxford Nanopore we did not use in this analysis):
| Accession | Description |
|---|---|
| SRR11140744 | veroSTAT-1KO |
| SRR11140746 | veroE6 |
| SRR11140748 | vero76 |
| SRR11140748 | swab |
We use the same Galaxy workflow described in Variation (opens new window) section of this repository.
The data are of exceptional quality:
# Quality of forward reads after fastp trimming
and there is a large number of reads mapping against NC_045512.2 (opens new window):
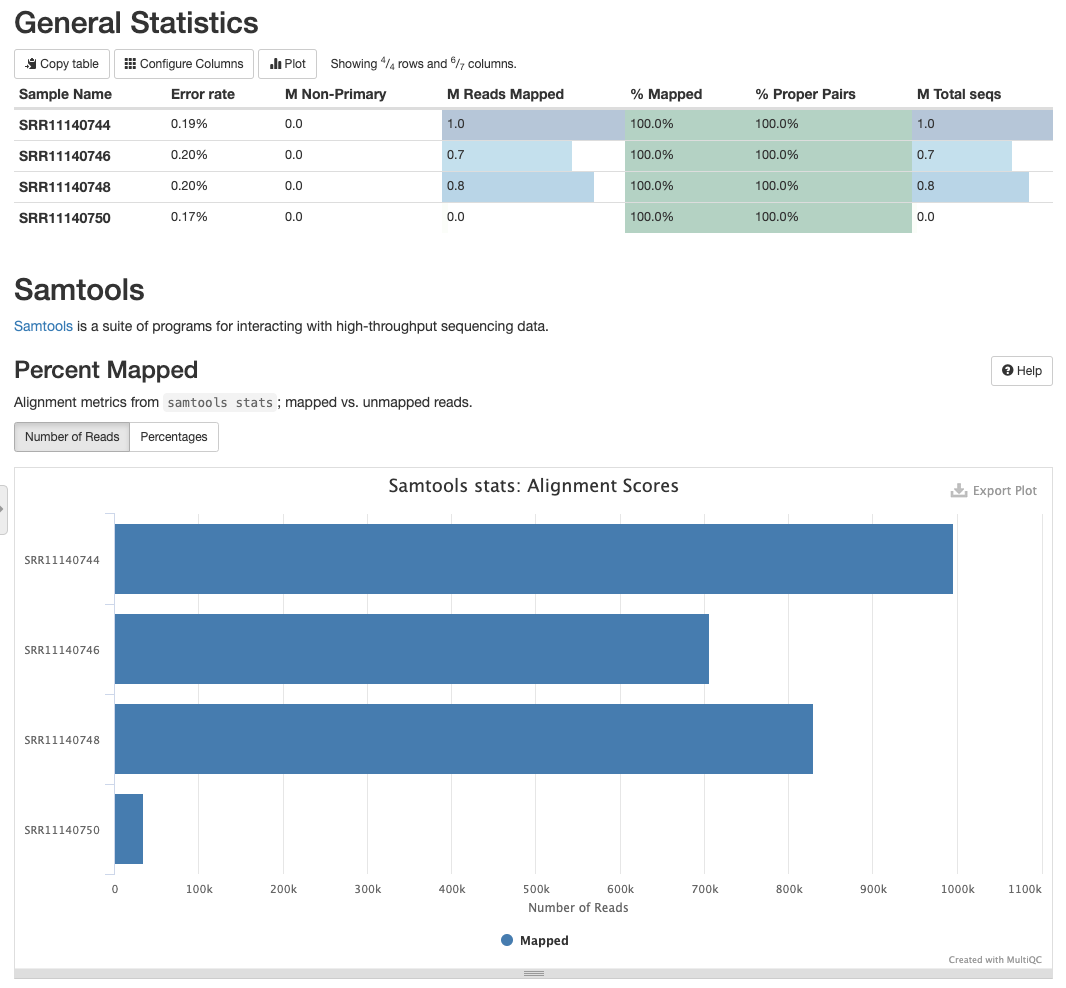
# Mapping statistics for SRR11140744, SRR11140746, SRR11140748, SRR1114050
Datasets SRR11140744, SRR11140746, SRR11140748 have high coverage and a large number of low frequency variants:
EFFECT . NON_SYNONYMOUS_CODING START_LOST STOP_GAINED \
Sample
SRR11140744 29.0 2304.0 3.0 25.0
SRR11140746 22.0 1604.0 1.0 20.0
SRR11140748 36.0 1899.0 2.0 24.0
SRR11140750 4.0 76.0 0.0 1.0
EFFECT SYNONYMOUS_CODING
Sample
SRR11140744 1594.0
SRR11140746 1120.0
SRR11140748 1315.0
SRR11140750 45.0
Restricting to only variants with frequency above 2% gives much more reasonable counts:
EFFECT . NON_SYNONYMOUS_CODING STOP_GAINED SYNONYMOUS_CODING
Sample
SRR11140744 0.0 4.0 0.0 5.0
SRR11140746 1.0 2.0 0.0 3.0
SRR11140748 0.0 6.0 0.0 3.0
SRR11140750 1.0 24.0 1.0 16.0
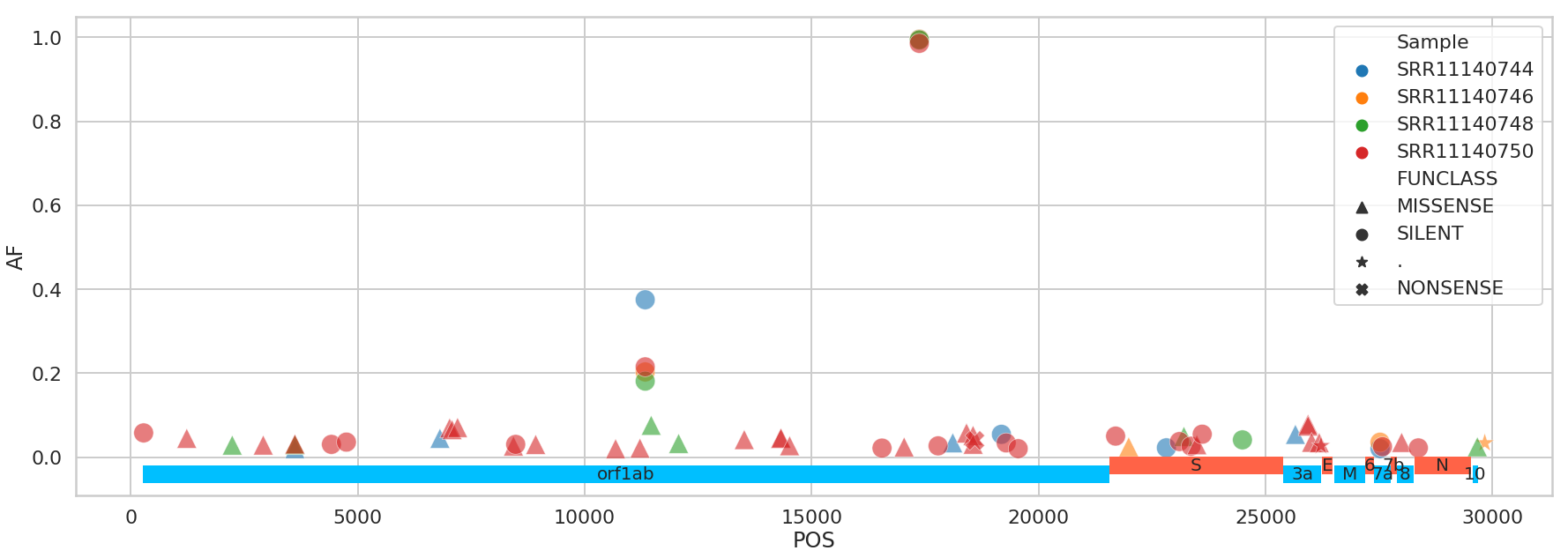
# Distribution of variants across COVID-19 genome
The list of variants is available from this repository. The file contains the following columns:
- Sample - Sample name
- CHROM - always the same value and can be ignored
- POS - position of variant (1-based as in VCF files)
- REF - Reference allele
- ALT - Alternative allele
- DP - Depth
- AF - Alternative allele frequency
- SB - Strand bias score as reported by
lofreq(Phred scaled result of Fisher's exact test; the lower - the better) - DP4 - Stranded counts: Ref +, Ref -, Alt +, Alt -
- IMPACT - Functional impact of the substitution
- FUNCLASS - Functional class of substitution
- EFFECT - Effect of the substitution
- GENE - Gene Name
- CODON - Codon with changes base capitalized
- type - S = SNP, I = Indel, M = MNP
For tools and workflows used in this analysis see Variation (opens new window) section of this repository.

